Abstract
Introduction: Few studies provide next-generation sequencing (NGS) based, clinical genomic profiling across lymphoma subtypes using tumor and matched normal samples to definitively identify somatic mutations and allele-specific copy number change. Here, we describe the somatic landscape of lymphoma samples from a large patient cohort using Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT) based panels.
Methods: Between 3/2015 and 11/2021, we assembled 2523 lymphoma samples sequenced with MSK-IMPACT. For patients with multiple samples, the first sequenced tumor sample was analyzed. Five variations of MSK-IMPACT panels containing a minimum of 400 genes were used. Samples were managed per institutional standard in our CLIA compliant Molecular Diagnostics Service laboratory (Cheng et al, J Mol Diagn, 2015). In some cases, multiple matched normal samples from blood, nail clippings, and saliva were used to definitively identify somatic alterations. Specimen information including tumor type, tumor purity, need for microdissection of the tumor region prior to nucleic extraction, and basic clinical staging and outcomes were collected. Tumor mutational burden (TMB) was calculated by dividing the number of mutations reported by MSK-IMPACT assay by the total genomic area where mutations were reported according to the version of the assay utilized. Genes were classified into oncogenic signaling pathways using published definitions (Sanchez-Vega et al, Cell, 2018). Genetic and treatment associations were assessed with Fisher's exact test and adjusted for multiple testing with the false discovery rate.
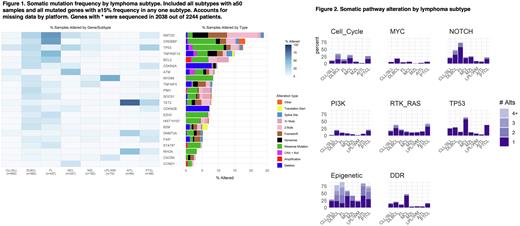
Results: The 2523 lymphoma samples included cases of CLL/SLL (n=600), DLBCL (n=560), FL (n=437), MCL (n=247), MZL (n=182), LPL/WM (n=70), AITL (n=80), and PTCL (n=68). Predominant tissue source were paraffin (58%), blood (25%), and bone marrow (10%). Lymph nodes (42%) were the majority of sequenced disease sites. Extranodal sites outside of blood and bone marrow represented 21% of samples. Most patient had advance stage disease at diagnosis: 68% DLBCL, 56% FL patients. Somatic mutation frequency and pathway dysregulation was assessed by lymphoma subtype (Figure 1 and 2). In CLL, the most common somatic mutations were TP53 (16%) and ATM (11%), however epigenetics (28%) was the most common altered pathway. Common DLBCL somatic mutations include KMT2D (31%), TP53 (25%), BCL2 (22%), CREBBP (21%), TNFRSF14 (20%), and EZH2 (11%). FL was characterized by epigenetic (89%) and Notch (73%) pathway dysregulation; single gene mutations of interest include EZH2 (21%), STAT6 (16%), TNFRSF14 (48%). MCL displayed alterations of ATM (41%), TP53 (28%), KMT2D (21%), notably no EZH2 mutations, and 47% had dysregulated DNA repair damage. The LPL/WM cases demonstrate 54% with MYD88, 16% with CXCR4 mutations consistent with NGS panels with higher false negative results for this disease (Kofides et al, Hemasphere, 2021). LPL/WM did not demonstrate alternations of genes classified in the MYC or cell cycle pathways. T cells lymphomas such as AITL and PTCL showed frequent mutations in TET2, DNMT3, RHOA; TP53 (32%) alterations were common in PTCL. We observed multiple mutations in somatic hypermutation genes such as KMT2D, BCL2, PIM1 and SOCS1, as well as TET2, a predominantly T cell lymphoma gene. Differential genes between treatment naïve and treatment exposed lymphoma samples included TP53 in CLL (q-value <0.001) and BCL2 (q-value <0.001); TP53 (q-value 0.008), CDKN2A (q-value 0.008), and CREBBP (q-value 0.002) in DLBCL. TMB varied among different histologies: ranged from CLL/SLL 1.8 (IQR 0.9, 2.8), DLBCL 10.2 (IQR 4.6, 14.8), FL 7.4 (IQR 4.6, 11.1), MCL 2.8 (IQR 1.8, 4.6), MZL 1.8 (IQR 0.9, 3.7), LPL/WM 0.9 (IQR 0, 1.8), AITL 3.2 (IQR 1.8, 4.6), and PTCL 4.6 (IQR 2.8, 6.7).
Conclusions: This series of 2523 lymphoma samples represents a large collection of mutational data across different histologic subtypes. Despite MSK-IMPACT panels not being a discovery panel, the assay serves to describe identified somatic mutations across lymphoma subtypes. Individual mutations represent a smaller lymphoma population, however recurrent genetic alterations converge on common oncogenic pathways which cumulatively represent a larger lymphoma population. This collection of data serves as a benchmark to design basket clinical trials based on a common dysregulated genetic pathway.
Disclosures
Batlevi:Roche/Genentech: Research Funding; Novartis: Research Funding; Janssen: Research Funding; Epizyme: Research Funding; Bayer: Research Funding; Autolus: Research Funding; Bristol-Myers Squibb: Other: Ownership / Equity Interests; Provision of Services; Xynomic: Research Funding; Seattle Genetics: Consultancy; GLG Pharma: Consultancy; Juno/Celgene: Consultancy; Life Sciences: Consultancy; Kite Pharma: Consultancy; Dava Oncology: Other: Provision of Services; ADC Therapeutics: Other: Provision of Services. Ptashkin:C2i Genomics: Current Employment. Falchi:Roche: Consultancy, Research Funding; Genetech: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Genmab: Consultancy, Research Funding. Horwitz:Shoreline Biosciences, Inc.: Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy; Yingli Pharma Limited and Tubulis: Honoraria; ADC Therapeutics: Research Funding; Affimed: Research Funding; Seattle Genetics,: Research Funding; Kyowa Hakko Kirin: Research Funding; Daiichi Sankyo: Research Funding; Millennium /Takeda: Research Funding; Celgene: Research Funding; Verastem/SecuraBio: Research Funding; Daiichi Sankyo: Membership on an entity's Board of Directors or advisory committees; SecuraBio: Honoraria; Cimieo Therapeutics: Honoraria; ONO Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Crispr Therapeutics: Research Funding; Affimed,: Consultancy; Kyowa Hakko Kirin: Consultancy; C4: Research Funding. Kumar:BridgeBio Pharmaceuticals: Current equity holder in publicly-traded company; Abbvie: Research Funding; Adaptive Biotechnologies: Research Funding; Celgene: Research Funding; Pharmacyclics: Research Funding; Seattle Genetics: Research Funding; AstraZeneca: Honoraria, Research Funding; Kite Pharma: Honoraria; Janssen: Honoraria. Lue:Epizyme: Consultancy; TG Therapeutics: Consultancy. Matasar:Rocket Medical: Consultancy, Research Funding; GlaxoSmithKline: Honoraria, Research Funding; Teva: Consultancy; Genentech, Inc.: Consultancy, Honoraria, Research Funding; ImmunoVaccine Technologies: Honoraria, Research Funding; Juno Therapeutics: Consultancy; Daiichi Sankyo: Consultancy; Merck: Consultancy, Current equity holder in private company; F. Hoffmann-La Roche Ltd.: Consultancy, Honoraria, Research Funding; Bayer: Consultancy, Honoraria, Research Funding; Seattle Genetics: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria; IGM Biosciences: Research Funding; TG Therapeutics: Consultancy; Karyopharm: Consultancy; IMV Therapeutics: Consultancy, Honoraria; Epizyme: Consultancy, Honoraria; ADC Therapeutics: Consultancy, Honoraria; Pharmacyclics: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; AstraZeneca: Consultancy. Moskowitz:SecuraBio: Research Funding; ADC Therapeutics: Research Funding; Biegene: Research Funding; Miragen: Research Funding; Seattle Genetics: Research Funding; Merck: Research Funding; Bristol-Myers Squibb: Research Funding; Incyte: Research Funding; Affimed: Honoraria; Imbrium Therapeutics L.P./Purdue: Honoraria; Janpix Ltd: Honoraria; Merck: Honoraria; Seattle Genetics: Honoraria; Takeda: Honoraria. Noy:Janssen: Research Funding. Palomba:Lygenesis: Honoraria; Pluto Therapeutics: Current holder of stock options in a privately-held company, Honoraria; Notch Therapeutics: Current holder of stock options in a privately-held company, Honoraria; Thymofox: Honoraria; GSK: Honoraria; Da Volterra: Honoraria; Ceramedix: Honoraria; Garuda: Honoraria; Nektar Therapeutics: Honoraria; Frazier Healthcare Partners: Honoraria; Rheos: Honoraria; Vor Biopharma: Honoraria; Seres: Current holder of stock options in a privately-held company, Honoraria, Research Funding; MustangBio: Honoraria; Kite: Honoraria; BeiGene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Synthekine: Honoraria; Novartis: Honoraria; BMS: Consultancy. Straus:Takeda Pharmaceuticals: Consultancy; Seagen: Consultancy. Vardhana:Immunai: Membership on an entity's Board of Directors or advisory committees; Koch Disruptive Technologies: Consultancy. Zelenetz:Beigene: Consultancy, Honoraria, Research Funding; Gilead/Kite Pharma: Consultancy, Honoraria; Genentech/Roche: Consultancy, Honoraria, Research Funding; Juno Pharmaceuticals: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; MBS: Consultancy, Honoraria; Pharmacyclics/Abbvie: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; AstraZeneca: Consultancy, Honoraria; Novartis: Consultancy, Honoraria. Arcila:Biocartis US, Inc: Consultancy; Clinical Care Options: Consultancy; Bristol-Myers Squibb: Consultancy; Invivoscribe: Consultancy; Janssen Global Services: Consultancy; PeerView Institute for Medical Education: Consultancy; Physicians’ Education Resource: Consultancy; AstraZeneca: Consultancy. Dogan:Peer View: Honoraria; Takeda: Other: Research Funding; Roche: Other: Research Funding; Loxo: Consultancy; EUSA Pharma: Consultancy; Seattle Genetics: Consultancy; Physicians’ Education Resource: Consultancy, Honoraria; Incyte: Consultancy. Salles:AbbVie, BeiGene, Bristol Myers Squibb, Celgene, Debiopharm, Epizyme, Genentech/Roche, Genmab, Incyte, Kite, a Gilead Company, Miltenyi, MorphoSys, Takeda, and VelosBio: Membership on an entity's Board of Directors or advisory committees; Roche/Genentech, Gilead Sciences, Janssen, Celgene, Novartis, MorphoSys AG, Epizyme, Alimera Sciences, Genmab, Debiopharm Group, Velosbio, Bristol-Myers Squibb, BeiGene, Incyte, Miltenyi Biotec, Ipsen, Kite, a Gilead Company, Loxo, Rapt: Consultancy; Roche/Genentech, Janssen, Celgene, Gilead Sciences, Novartis, AbbVie, MorphoSys AG, Amgen, Bayer, Epizyme, Regeneron, Kite, a Gilead Company: Honoraria. Zehir:Astrazeneca: Current Employment; Arcus Biosciences, Inc.: Other: Ownership / Equity Interests. Younes:Astrazeneca: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal